Identifying the right role for you
Figuring out where you fit in pharma isn’t always easy.
Whether you're trying to break in, switch lanes, or just make sense of job descriptions that feel like another language, it can feel overwhelming. There are so many roles, titles, and pathways, and it’s not always clear what’s right for you.
That’s where we come in.
We’re here to help you cut through the noise, understand what different roles actually involve, and figure out where your skills, interests, and career goals line up best. Whether you’re just starting out or looking for your next big move, our goal is to make the pharma world feel a lot less confusing—and a lot more open.
Take the quiz to help you to identify which roles you might be best suited to.
Scientific Communications
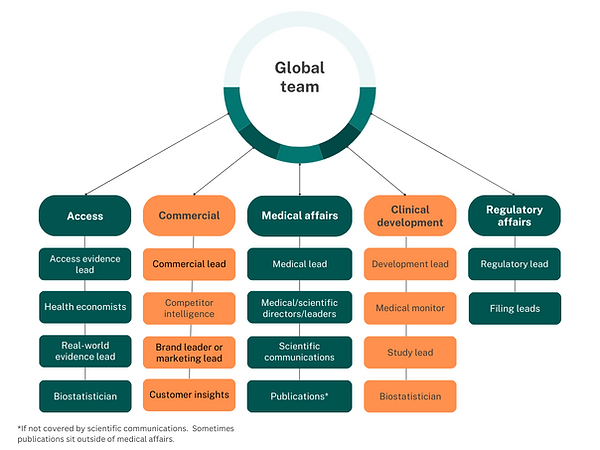
The role of the scientific communications teams can vary across companies, but typically they are responsible for driving the scientific communication strategy, publications strategy (depending on the company), scientific narrative and scientific statements. Scientific communication professionals really understand the audiences and which messages will resonate, as well as which channels they use.

Global commercial
The global commercial or marketing function is responsible for shaping and delivering the brand strategy at a global level. This includes developing high-impact content and tools that can be rolled out or adapted by local teams. It’s a role that sits at the intersection of science, business, and creativity—combining customer insight, data, and storytelling. Global marketers often lead launch planning, work closely with cross-functional teams (medical, market research, regulatory, supply chain), and play a key role in defining how a brand shows up in the world. They may also support sales forecasting, competitive analysis, and training—essentially helping bring the strategy to life at scale.

Global Medical Director
The role of a Medical Director in medical affairs is wide-ranging and strategically important. It goes beyond data review—Medical Directors shape medical strategy, guide evidence generation, and build the scientific narrative that supports both clinical practice and business goals. They lead cross-functional collaboration, engage with external experts, and equip internal teams with the insights and tools they need to drive meaningful HCP and patient engagement. Whether it’s authoring publications, supporting launches, or shaping global education, Medical Directors are central to making sure the science translates into real-world impact.

Medical Science Liaison (MSL)
MSLs are scientific experts who act as a bridge between the company and external healthcare professionals. They engage in peer-to-peer conversations with key opinion leaders (KOLs), sharing clinical and scientific data in a non-promotional way. MSLs support medical strategy by gathering field insights, shaping evidence generation, and ensuring the right data reaches the right people at the right time. They play a crucial role in translating complex science into meaningful clinical conversations.

New to Medical Affairs and want to learn more?

I’ve partnered with Wolf Medical to share their Medical Affairs Fundamentals CPD-accredited course. It’s designed to give you a solid grounding in the core skills and knowledge needed to succeed in a Medical Affairs role, from stakeholder engagement to managing advisory boards.
Price: €615
Global Publications Manager
The role of a publications manager is to ensure that data from clinical trials and research studies is communicated clearly, accurately, and in a timely way through scientific publications. In some companies, this role sits within medical affairs and scientific communications, while in others it exists as a standalone function. Publications managers work cross-functionally with internal teams, external experts, and agency partners to plan, develop, and deliver abstracts, posters, and manuscripts for congresses and peer-reviewed journals. The role also often involves navigating complex processes, ensuring compliance, and increasingly, exploring innovative formats like infographics and dashboards to expand the reach and impact of scientific data.

Market Access
Market Access is the function responsible for ensuring that a medicine or device doesn’t just get approved—but actually reaches patients through healthcare systems. It involves shaping how a product is perceived, valued, and ultimately funded by payers and other key stakeholders. Work typically starts years before launch and includes activities like value messaging, developing global value dossiers, generating health economics data, and supporting pricing and reimbursement strategies. The function blends strategic thinking with hands-on implementation and often overlaps with health economics, policy, and payer engagement. It's a crucial bridge between clinical development, commercial strategy, and real-world patient access.
HEOR/VEO
Health Economics and Outcomes Research (HEOR), sometimes referred to as Value, Evidence, and Outcomes (VEO), is a vital discipline in the pharmaceutical and biotech industries that focuses on understanding the value of healthcare interventions. HEOR combines data on clinical outcomes, patient quality of life, and economic impact to help inform decisions around drug pricing, reimbursement, and access. By generating real-world evidence and modelling cost-effectiveness, HEOR supports payers, policymakers, and healthcare providers in making data-driven choices that balance innovation with affordability—ensuring patients can access treatments that truly make a difference.
Project/Program Manager
The role of a project or program manager in pharma is all about keeping complex, high-stakes initiatives on track. Whether it’s a clinical trial, R&D development, or a global launch, they work across teams to align timelines, budgets, and goals—ensuring nothing falls through the cracks. They’re often the glue between functions like regulatory, medical, and commercial, managing risk, unblocking issues, and helping teams move faster and smarter. It’s a role that combines planning and people skills with a strong understanding of the scientific and business context.

External/Group Communications
External and group communications professionals play a critical role in shaping how a company shows up in the world, whether that's to policymakers, media, or the public. In pharma, this team is often responsible for translating complex science into compelling narratives, aligning internal and external messaging, and driving communications that influence public perception and policy. They’re deeply embedded in corporate strategy, public affairs, and reputation management. A strong external communications lead understands how to humanize science, engage stakeholders across sectors, and build trust through consistent, purpose-led messaging. Depending on the company, the remit may also include government affairs, campaign planning, media relations, and thought leadership platforms.

Other guides coming soon.

Struggling to land a biotech/pharma job? The industry can be so competitive you are not alone. Grab your free copy of the Breaking into Biotech guide
What's inside:
Discover the best entry-level roles
Learn key responsibilities & career paths
Get pro tips to optimize your job search
1 hr
120 Swiss francs